The Pros and Cons of Platelet-Rich Plasma in Dentistry
Key Points:
• Platelet-Rich Plasma (PRP) is not FDA approved. It is an “off-label” therapy.
• PRP is considered safe.
• There is no standardization of PRP treatments, making it difficult to determine how it heals or encourages healing.
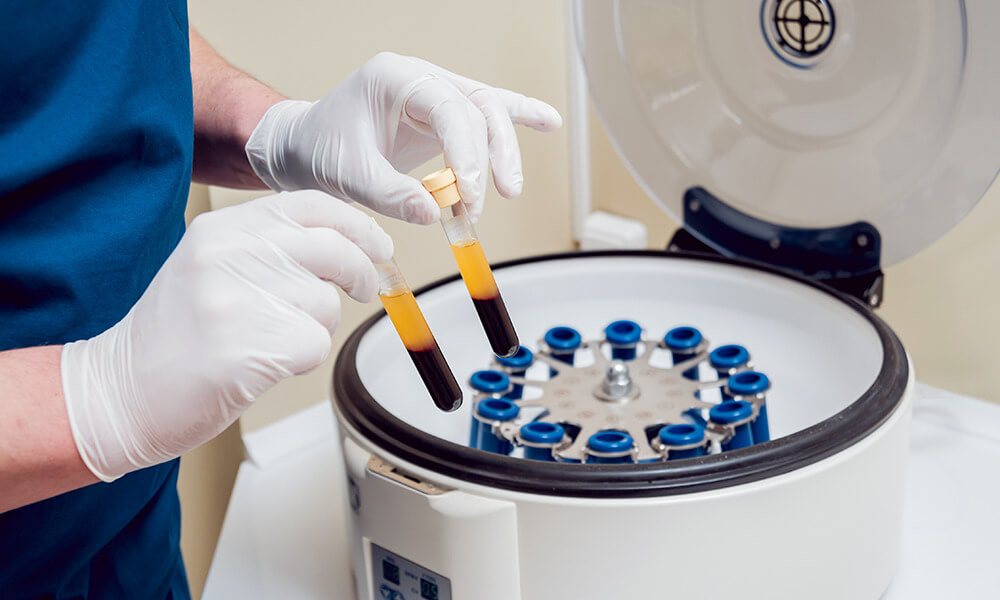
If you regularly work with an oral surgeon, you’ve probably heard the term Platelet-Rich Plasma or “PRP.” Put simply, PRP is a technique where blood is drawn from a patient and spun in a centrifuge to produce a clot composed of the patient’s own platelets. This clot is then placed into a wound or surgical site to stem the flow of blood and promote healing. In short, a clinician removes the red blood cells from whole blood.
PRP is an autologous cell therapy. This just means that cells are used on the same patient they came from.
What is PRP and How is it Regulated?
PRP is a relatively simple procedure that is favored in clinical practice due to its safety and perceived efficacy. Because it is drawn from a patient’s own blood and is similar to a previously cleared therapeutic, it has so far bypassed the lengthy and expensive process of FDA approval for biologics.1 PRP is not FDA approved, though it is cleared legally for specific therapeutic uses, and therefore guidelines must be followed to adhere to FDA regulations.1
A 2018 review of the economics and regulatory outcomes for PRP describes the treatment’s 5-to-10-year outlook as enormous, citing “between 380 million and 4.5 billion (USD)” in growth.1 Procedures may start at a minimum of $500 and can be thousands of dollars.1
What this means in short is that PRP is an expensive treatment lacking in proven clinical trial data and is not paid for by insurance.1 This may not sound like a promising description, but it doesn’t mean the treatment is ineffective or not worth the money depending on its application.
PRP is regulated based on certain qualities of how it is collected from the patient and the number of platelets per volume.1 It often contains a unique combination of leukocytes (immune cells/white blood cells), platelets (cell fragments without a nucleus), and fibrin (fibrous protein necessary for clotting).1
Why Clinical Trial Data is Limited for PRP
The large variation in techniques used for the collection of PRP, as well as inherent differences in composition that can be attributed to individual patient differences, make it supremely difficult to study the efficacy of the treatment.1 It also makes it very challenging to compare different treatment styles. It is complicated to design a study with the necessary “statistical power” to draw a valid conclusion from the data. This is true of many biologics or stem-cell based therapies. How do you determine that the effect you are witnessing is truly a result of the cell-based therapy? Moreover, what part of the therapy is generating the effect? Cells are unpredictable.
In order to study the effect of PRP therapeutically, it would be necessary for multiple studies with large sample sizes to use the exact same materials and experimental conditions, all the way down to the exact collecting tube and centrifugation speed.1 Clear and replicable methodology (the exact steps taken, in what order, with what materials, and for what duration) is often missing in scientific literature due to fears over proprietary information or lack of thorough reporting.
PRP is a Mixture of Many Different Therapeutic Substances
When trying to understand or tease out how PRP effects the surrounding tissue, it’s helpful to think of the clot as an intentionally overcooked minestrone. This soupy, gelatinous mixture was made by a brilliant chef with a few screws loose. We know there are tomatoes and carrots and potatoes, with some vegetable broth and a bit of salt, but some of the ingredients are difficult to identify. Did she throw in a dash of dill? Is it the pepper that’s leaving such a strong aftertaste? Does the minestrone taste good mainly because of the tomato? The tomato is everywhere, so it’s easy to differentiate. But that can’t possibly be the entire picture contributing to such a delicious soup du jour.
PRP is infinitely more complicated than a well-made minestrone. It contains growth factors, AKA a protein or hormone that regulates cell behavior, such as proliferation or healing.1 But it also contains the immune cells mentioned earlier. And ions. And many other molecules and proteins with diverse purposes in the body. It’s hard to offer a therapy to someone if you don’t fully understand why it works. You know the soup is tasty, but what about this exact combination of ingredients is making it better than the one you make at home from a can?
So far, PRP has been deemed relatively safe.1 It is considered an “off-label” treatment that was cleared, but not approved, by the FDA under the 510(k) substantial equivalency pathway.1 This pathway allows transplanted human tissue or other medical devices to be cleared if it similar enough to a prior cleared device.1 There are pros and cons to off-label use of drugs and biologics.1 One major con is that there is a dearth of scientific evidence in humans explaining why and how the biologic works.1
PRP in Oral Surgery
PRP is used for a variety of orthopedic purposes. Oral surgery is just one of the many ways in which this therapy is leveraged. A 2013 study of PRP in dental surgery found mixed results in the literature.2 The goal of PRP for tooth extraction is to manage the bleeding and pain of extraction sockets while encouraging bone formation.2 Some studies found improved benefits to post-operative pain, bleeding, and bone healing, whereas other studies found no significant effects of PRP, especially long-term for bone growth.2 Overall, the review indicates that there is evidence of PRP being effective for soft tissue but not for bone regeneration.2
The body of knowledge regarding Platelet-Rich Plasma will continue to grow in the next decade. Over this period of time, perhaps the FDA approval process will be fruitful and standardized treatments will make their way onto the market.
References:
- Jones IA, Togashi RC, Thomas Vangsness C. The Economics and Regulation of PRP in the Evolving Field of Orthopedic Biologics. Curr Rev Musculoskelet Med. 2018;11(4):558-565. doi:1007/s12178-018-9514-z
- Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immunity & ageing. 2013;10(1):23-23. doi:1186/1742-4933-10-23
Related Course
TMD & Orofacial Pain: Managing Complex Patients
DATE: June 24 2026 @ 8:00 am - June 28 2026 @ 1:00 pmTMD patients present with a wide range of concerns and symptoms from tension headaches and muscle challenges to significant joint inflammation and breakdown. Accurate thorough diagnosis is the first step…
Learn More>






